by Dr. Maya Panisset
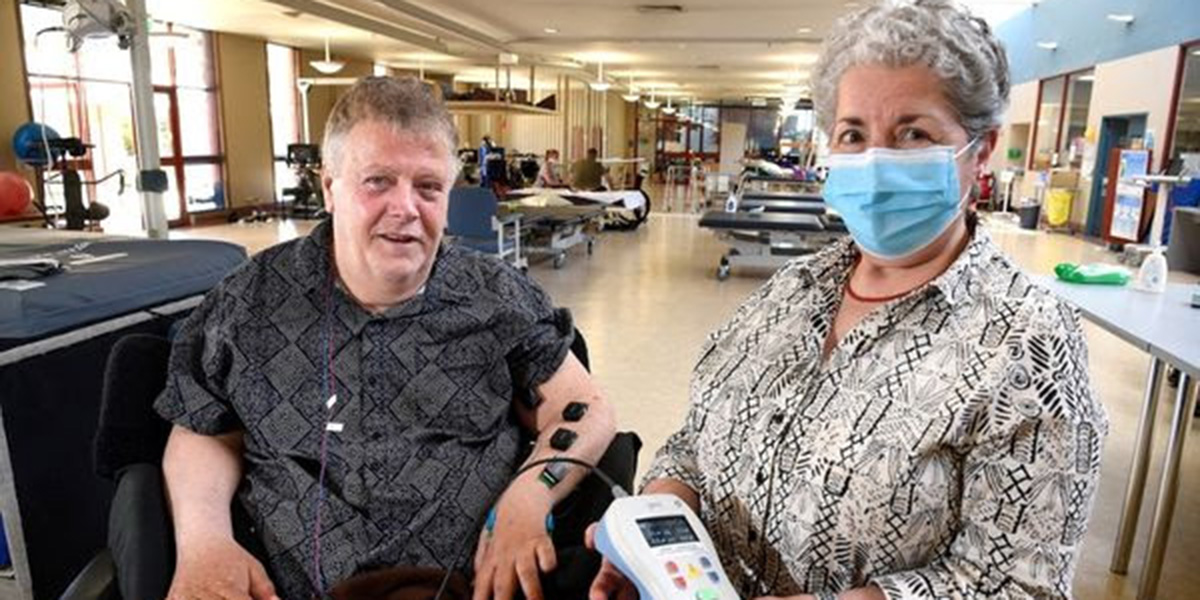
An exciting clinical trial of transcutaneous electrical spinal cord neuromodulation (TESCoN) is underway at the Royal Talbot Hospital in Kew, Victoria.
Researchers from the University of Melbourne are looking for volunteers to participate. The trial involves four weeks of daily (Mon-Fri) TESCoN plus physiotherapy for upper limb function and sitting balance. So far, over 20 people have completed the trial, but the team are hoping to boost recruitment from the community by providing a sneak peek into the trial experience.
TESCoN is a clinical trial, so while there are no promises of recovery or functional improvements, every participant so far has achieved something positive out of the trial, and each participant brings us closer to making this promising technology available to everyone.
What is TESCoN?
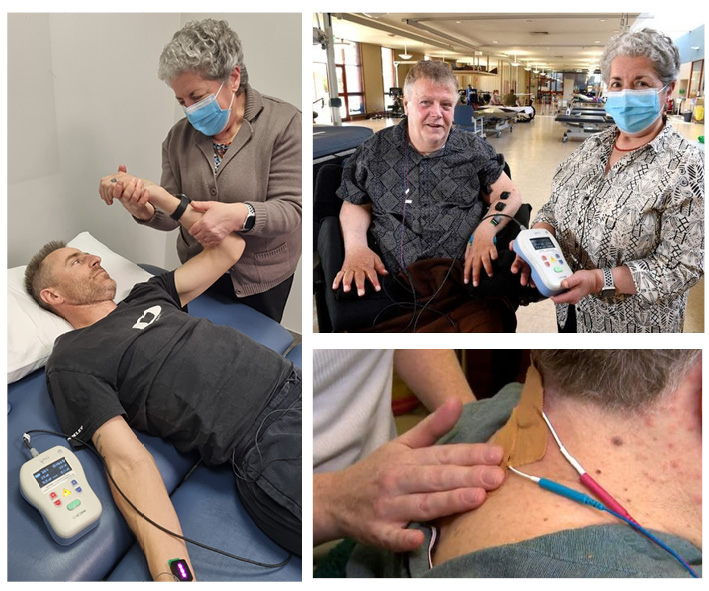
Transcutaneous electrical spinal cord neuromodulation (TESCoN) is a non-invasive form (not requiring surgery) of neuromodulation. Electrodes are placed on the skin over the spinal column and used to stimulate the spinal cord via an electrical current. This electrical current targets spinal cord pathways that have been spared from the injury, but are not working normally.
Modulation of these spinal networks by TESCoN raises the baseline excitability level so that the spinal circuitry below the injury requires smaller levels of excitation to start working.
Intensive rehabilitation combined with TESCoN enables a change in the connections within and between the networks involved in controlling movements (called activity-dependent plasticity), resulting in improved function.
For a more comprehensive understanding of how the therapy works, check out this article.
Who’s eligible?
- People with a cervical spinal cord injury
- Either 3-6 months after injury, or at least 1 year post injury
- Between 15-75 years old
- No other injuries
- No other neurological conditions
- No contractures in hands & arms
- No tendon or nerve transfer surgery
What’s it like to take part in the trial?
Participants reported that the treatment was challenging at times, both mentally and physically, but some also described that it gave them “a glimmer of hope.” Overall, people felt they were in good hands with the highly knowledgeable research team and felt it was an overall positive experience.
What sort of improvements have been seen?
Participants with chronic incomplete injuries have reported having better strength, motor control, and decreased spasticity, all of which also translated into functional gains. This group also reported sensory changes, like an awareness of a full bladder, and being able to feel if a coffee cup is hot (an important safety issue!)
They described gaining “better control of the(ir) movements (and) more range. Which helps with everything.” Others noted that better fine motor control and sensation helped them at work.
What about people with chronic complete injuries?
Improvements in this group have varied from person to person, and the team need more people to come and give it a go. They recognise it can be difficult to organise carers and accommodation for such an intense program, so the trial provides $100/day for participants living in the community, to help with costs of transportation, etc.
Is it worth the trouble?
One recent participant saw some amazing gains in fine motor skills and sitting balance, which also helped their breathing. Some have improved their bed mobility; now being able to roll over in bed and do sliding board transfers has made them feel more independent. Improved trunk stability has helped everyday tasks like being able to hold a cup with two hands.
Would they recommend the program to others in the AQA community?
“TESCoN has been nothing but beneficial, both physically and mentally. Before TESCoN, I didn’t think I would gain any further improvements. But now I am able to do so much more with my hands; it has given me hope.” – Participant
“I went into the program with an open mind, not expecting anything magic; but I was really impressed. I really enjoyed the experience. The gains I made were definitely worth the investment.” – Participant
Find out more and register your interest here or contact:
Dr. Maya Panisset
0405 027 127
The TESCoN trial is led by Professor Mary Galea from the University of Melbourne, with funding by the Australian Medical Research Future Fund.
This project is approved by the Austin Health Human Research Ethics Committee
- July 18, 2024